Answer
Mar 04, 2025 - 12:56 PM
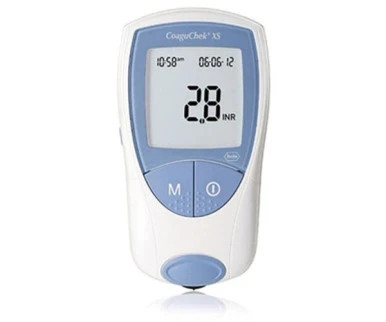
Yes, CoaguChek systems, which are used for monitoring blood coagulation levels, are considered waived under the Clinical Laboratory Improvement Amendments (CLIA) in the United States. This means they are categorized as simple tests with a low risk for an incorrect result, allowing them to be used in a variety of healthcare settings with less stringent regulatory requirements.